Assay & Application Notes
Validating Absorbance 96 Application for XTT Assay Using the instaCELL® Cytotoxicity Kit (XTT)
In collaboration with acCELLerate GmbH

Key highlights
- Absorbance 96 is a robust and effective microplate reader for conducting XTT cell viability assays.
- Integration of 96 detection units in Absorbance 96 enables fast and simultaneous measurement of all the wells — beneficial for time-sensitive, cell-based kinetic studies.
- The use of the instaCELL® Kit's assay-ready cells reduces variability, enhancing the accuracy and reliability of the XTT assay.
Introduction
Cell-based models have emerged as indispensable and well-established tools across various scientific domains, including basic research, target testing, and drug discovery (1). Notably, these cell-based assays have gained significance in the pharmaceutical sector. For instance, in toxicology, cell-based assays offer a promising alternative to animal testing and experimentation (2). In GLP/GMP testing, the requirement for accuracy and reproducibility of cell-based assays is of particular importance, with the application of assay-ready cells proving to be highly advantageous. These cells are readily available for experimentation, eliminating the need for prior cultivation, and can be directly employed as reagents in various assays.
One of the most widely used cell-based assays is the XTT assay, which enables the quantification of cellular metabolic activity, serving as a reliable indicator of cell viability, proliferation, and cytotoxicity. This colorimetric assay is based on the reduction of a yellow tetrazolium salt (sodium 3 -[1- (phenylamino carbonyl) - 3,4 - tetrazolium] - bis (4 -methoxy 6 - nitro) benzene sulfonic acid hydrate or XTT), by metabolically active cells. The reduction process yields an orange formazan dye, which remains soluble in an aqueous solution and can be directly measured using a microplate reader (3, 4). As the number of viable cells increases, the activity of mitochondrial dehydrogenase also increases in the sample, resulting in a proportional increase in the orange formazan formation. And this phenomenon can be accurately monitored by measuring the absorbance readouts.
This application note aims to present the validation of Absorbance 96 as a suitable microplate reader for performing XTT cell viability assays with instaCELL® Cytotoxicity Kit(SF020-02, acCELLerate). The kit provides all necessary reagents (assay-ready L-929 cells, recovery buffer, assay medium, assay plates, XTT viability assay reagent, and suitable positive and negative controls) for performing the XTT assay. The results obtained highlight the streamlined XTT assay workflow made possible by using the instaCELL® Cytotoxicity Kit and the kinetic measurement capability of the Absorbance 96 microplate reader. This study was performed in collaboration with acCELLerate GmbH.
Results
A comprehensive set of tests was undertaken to assess critical parameters essential for cell-based assay workflows to evaluate the suitability of the Absorbance 96 microplate reader for such assays. This study specifically focused on the XTT cell viability assay. It included assessing factors such as well-to-well variability and the measurement accuracy of Absorbance 96 for the XTT assay.
Determining well-to-well variability
Absorbance 96 is a solid-state microplate reader equipped with 96 detection units, enabling simultaneous measurement of the absorbance values of all 96 wells when performing XTT assays or other colorimetric methods. Due to this unique optical setup, assessing the measurement accuracy and variability across the 96-well plate is crucial. To test this, 30.000 L-929 cells were seeded and cultivated into each well of a 96-well plate. The cells were then incubated for 24 hours at 37°C in a humidified atmosphere. Subsequently, tetrazolium salt (XTT) was added to the cells. To minimize the impact of cell culture conditions and cell size on the absorbance readout, the resulting supernatant from all 96 wells was pooled and dispensed into a new 96-well plate (5). The plate was then inserted into the Absorbance 96, and the optical density (OD) at 492 nm was measured (Fig. 1).
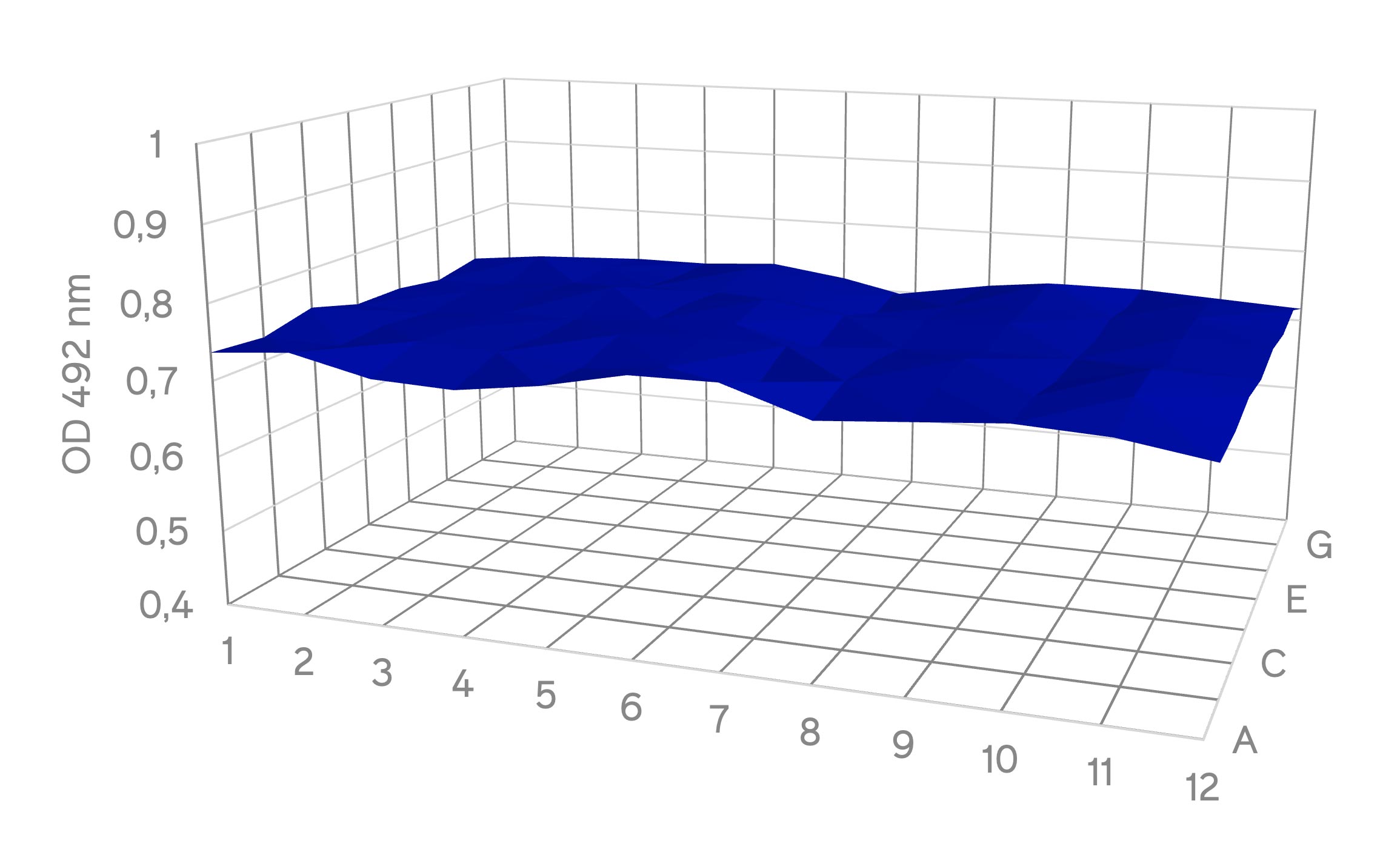
Figure 1: Surface plot of absorbance measurement at 492 nm across different wells in a 96-well plate with XTT assay
The results show a uniform distribution of absorbance measurement across the 96-well plate, with a significantly low standard deviation (SD) of 1.2 %. This is well within the expected range for cell-based assays, considering inherent factors that might affect variability in different wells, such as the number and growth conditions of cells per well or edge effects (6). Also, no outliers were detected, signifying consistent absorbance measurements and highlighting low well-to-well detection variability in Absorbance 96 for measuring absorbance kinetics across the whole 96-well plate. Accurate measurement in a cell-based assay is crucial, particularly when employing various experimental conditions and seeding cells in a 96-well plate. Minimizing well-to-well variability becomes critical to enhancing measurement accuracy.
Measuring XTT assay kinetics
Before progressing with subsequent evaluation steps, determining optimal cell seeding density was crucial to ensure XTT assay validity and measurement accuracy. So, L−929 cells were seeded in a 96−well plate at different densities ranging from 1.000 to 100.000 cells per well. Subsequently, the cells were incubated at 37°C for 24 hours to promote adhesion. Following the incubation period, XTT was added to quantify the metabolic activity of the cells. The plate was then inserted into Absorbance 96, and absorbance kinetics were monitored over 4 hours to determine the optimal incubation time and cell seeding density for the XTT assay (Fig. 2). 3E+04 cells were chosen per well because of the best signal-to-noise ratio with a simultaneous almost linear slope over the entire incubation period.
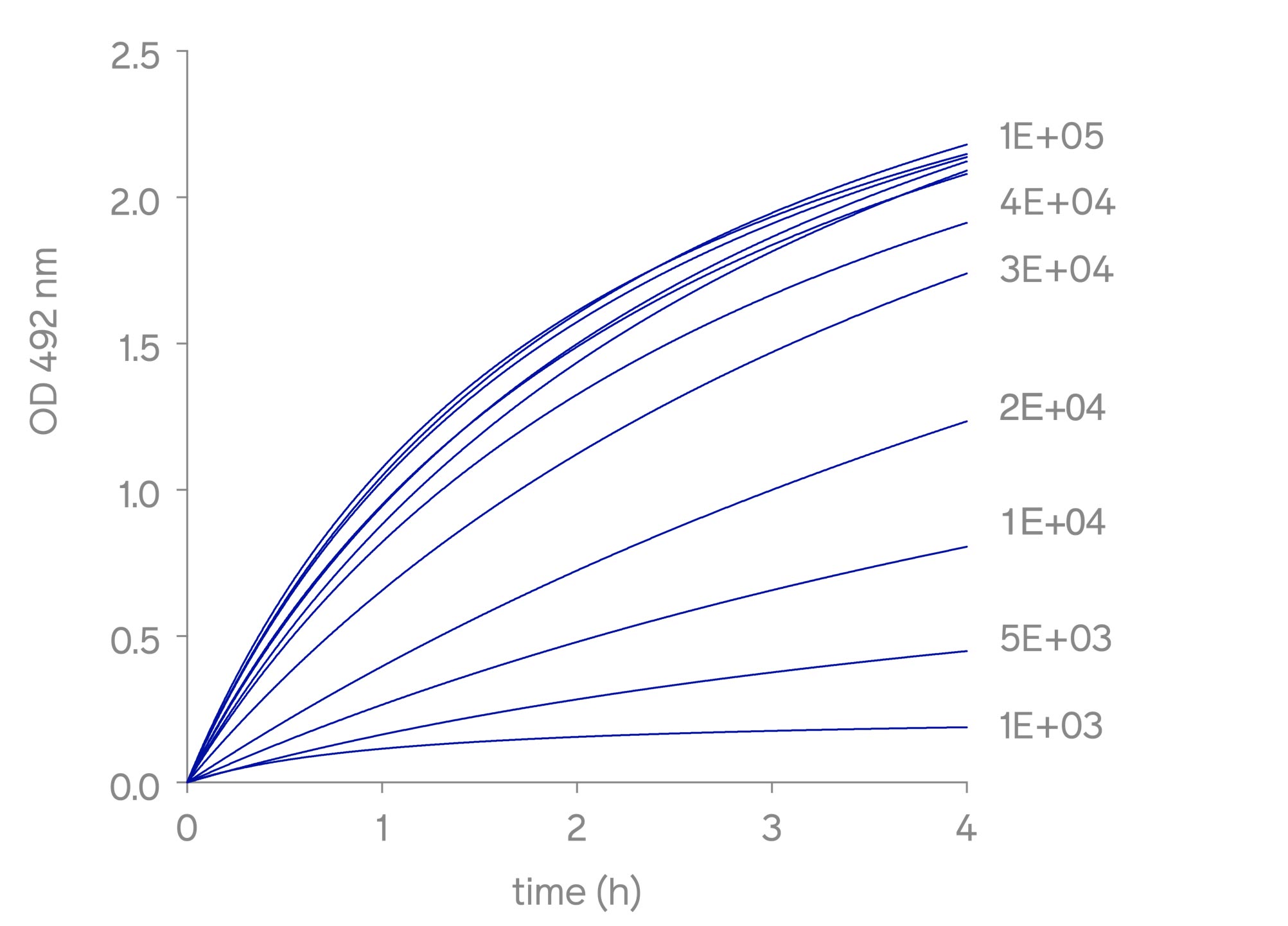
Figure 2: Kinetic analysis of XTT metabolization over time with different cell densities
Evaluating XTT assay accuracy
Properly standardized cell culture protocols and procedures are essential for reproducible and routine cell use. To minimize the variations and discrepancies related to cell culture results, using assay-ready cells has proven to be a valuable approach. Assay-ready cells are cryopreserved at a high viability and functional state, allowing their direct use in assays without prior cultivation, thereby minimizing culture protocol-based variability.
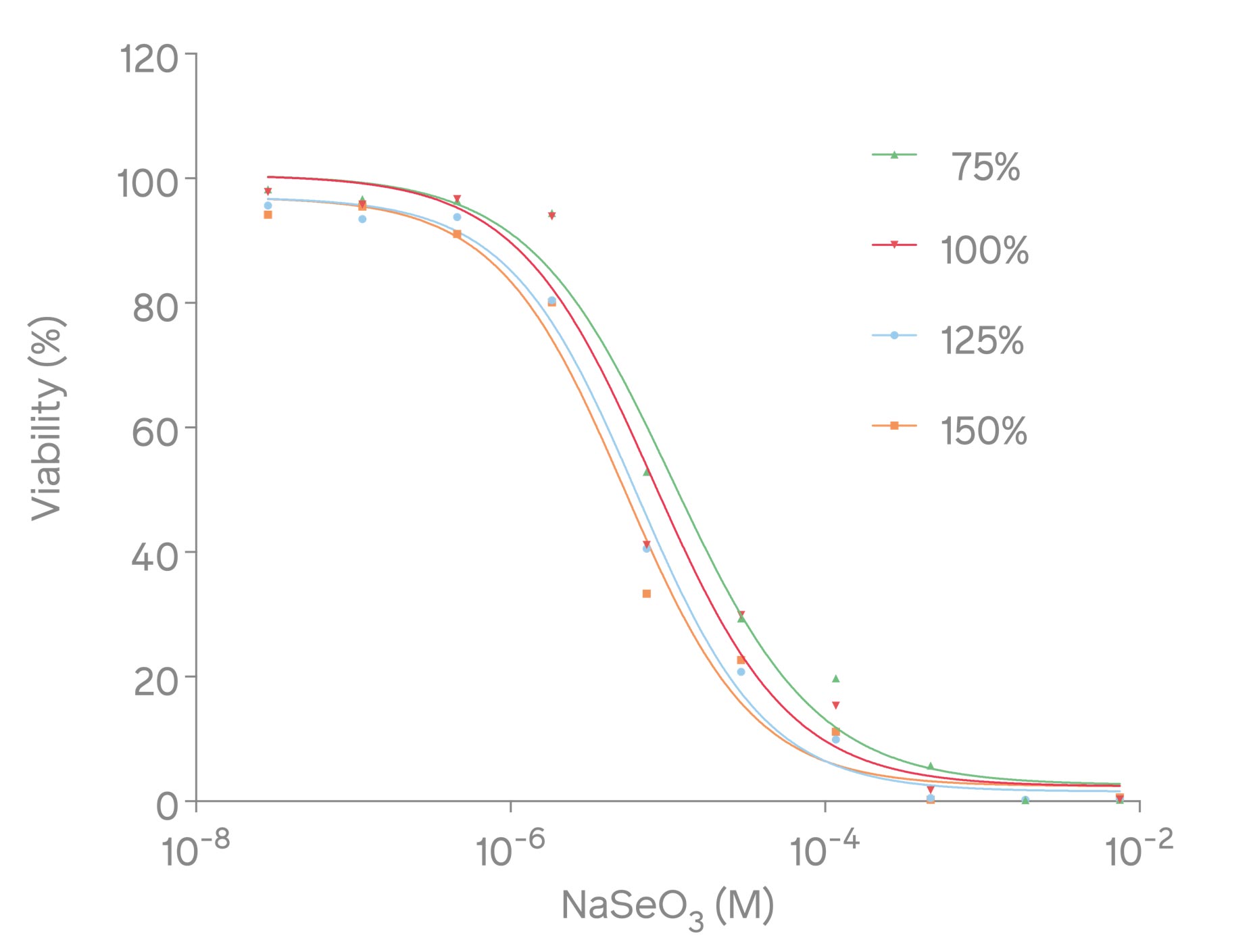
Figure 3: Dose-dependent response curve of sodium selenite with XTT assay
To examine the accuracy of the XTT assay, a dose-dependent response curve was constructed using sodium selenite, a toxic reference compound. Studies have shown that sodium selenite induces cell death and apoptosis by producing superoxide in mitochondria and activating the mitochondrial apoptotic pathway (7). To do so, assay-ready L-929 cells were thawed at 37°C in a water bath, and the cell suspension was then transferred into 8 mL of prewarmed RPMI medium. Subsequently, the cells were pelleted by centrifuging at 80xg for 3 minutes, which was then resuspended in a 10 ml assay medium (RPMI 1640, 20 % fetal bovine serum, 2 mM L-Gln). Afterward, 20.000 cells per well were seeded into a 96-well plate. To evaluate the cells' response to varying sodium selenite concentrations, four sets of serial dilutions (75 %, 100 %, 125 %, and 150 %) of the nominal concentration were prepared and added to the cells and incubated at 37°C for 24 hours. After incubation, XTT was added to the cells and incubated for 4 hours. The metabolic activity, indicative of cell viability, was then determined by measuring the absorbance at 492 nm with Absorbance 96 (Fig. 3).
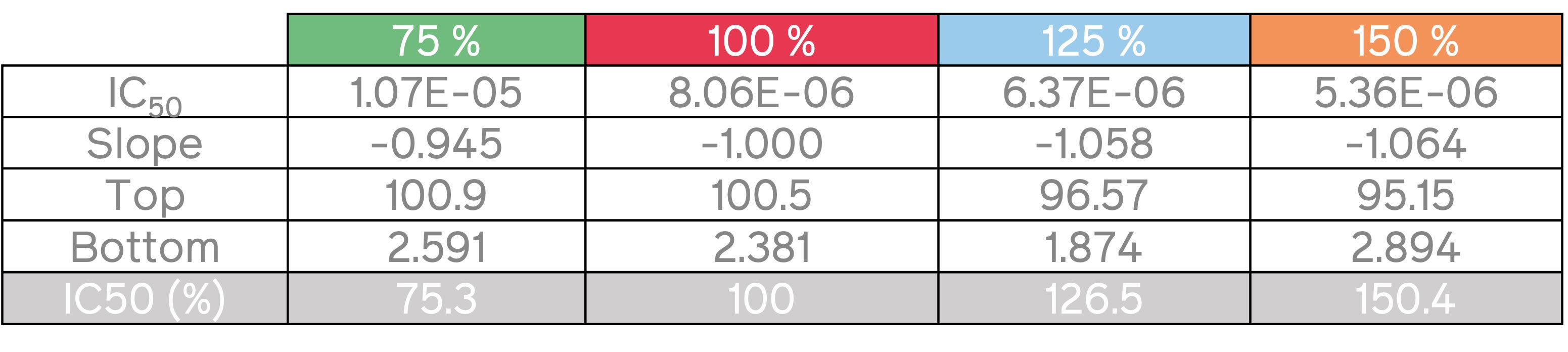
Table 1: Tested serial dilutions of the nominal concentration of sodium selenite to determine the IC50 values and access the XTT assay accuracy
The IC50 value was calculated for each of the four sets of dilutions (Table 1). The IC50 values closely corresponded to the calculated concentrations of the dilutions, thereby validating the accuracy and reliability of the assay method. These results highlight the suitability of Absorbance 96 as a reliable microplate reader for performing cell-based assays such as XTT assay.
Discussion
The XTT assay is a valuable method for studying changes in cell number and metabolic activity, providing insights into cell viability, proliferation, and cytotoxicity. The results obtained in this application note confirm the effectiveness and accuracy of Absorbance 96 for conducting an XTT assay. With its design incorporating 96 detection units, Absorbance 96 facilitates rapid readout from all the wells in a 96-well microplate, allowing for fast measurements of the absorbance kinetics of the samples. Additionally, Absorbance 96 demonstrates the ability to accurately detect XTT metabolization across a range of cell densities, from 1.000 to 100.000 cells, highlighting its suitability for various cell-based assays. The need for extensive cell culture manipulation and complex setups associated with other microplate readers can introduce variability and negatively impact cell-based assay outcomes. However, assay-ready cells provided with instaCELL® Cytotoxicity Kit combined with Absorbance 96 offers a user-friendly workflow for performing accurate XTT assays.
References
1. Hirsch C, Schildknecht S. In vitro research reproducibility: Keeping up high standards. Front Pharmacol. 2019 Dec 10;10:492837.
2. Zink D, Chuah JKC, Ying JY. Assessing Toxicity with Human Cell-Based In Vitro Methods. Trends Mol Med. 2020 Jun 1;26(6):570–82.
3. Paull KD, Shoemaker RH, Boyd MR, Parsons JL, Risbood PA, Barbera WA, et al. The synthesis of XTT: A new tetrazolium reagent that is bioreducible to a water-soluble formazan. J Heterocycl Chem1988 May 1;25(3):911–4.
4. Talekar M, Kendall J, Denny W, Jamieson S, Garg S. Development and evaluation of PIK75 nanosuspension, a phosphatidylinositol- 3-kinase inhibitor. Eur J Pharm Sci. 2012 Dec 18;47(5):824–33.
5. Stevenson K, McVey AF, Clark IBN, Swain PS, Pilizota T. General calibration of microbial growth in microplate readers. Sci Reports 2016.
6. Mansoury M, Hamed M, Karmustaji R, Al Hannan F, Safrany ST. The edge effect: A global problem. The trouble with culturing cells in 96-well plates. Biochem Biophys Reports. 2021 Jul 1;26:100987.
7. Xiang N, Zhao R, Zhong W. Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother Pharmacol. 2009 Jan;63(2):351–62.