Assay & Application Notes
Enhancing Lab Automation: Validating Absorbance 96 Automate Integration with Eppendorf epMotion® for Automated Cell-Based Assays
In collaboration with Eppendorf SE

Key Highlights
- Evaluation of the Absorbance 96 Automate compared with another benchtop microplate reader revealed comparable results in reproducibility, sensitivity, and linearity across the dynamic range.
- Seamless on-deck integration of the Absorbance 96 Automate with the epMotion® liquid handling system streamlines the cell-based assays in an automatic workflow.
- Results confirm the effective application of Absorbance 96 Automate in an automated workflow for cell-based assays with high precision.
Introduction
The lab automation of microplate reading on liquid handling systems significantly advances laboratory workflows. This integration seamlessly combines the precise liquid handling capabilities of liquid handlers with the analytical readout efficacy of the microplate reader. This integrated approach enhances experimental throughput by facilitating simultaneous measurements of multiple samples, allowing researchers to analyze cell cultures more efficiently and expeditiously. The streamlined workflow mitigates potential manual errors associated with pipetting, cell seeding, and data recording, thereby ensuring the reliability and reproducibility of assay results, which is crucial in cell-based assays. This solution proves particularly advantageous in cell-based assays where precision, speed, and reproducibility are paramount for optimizing experimental processes. Consequently, it empowers researchers to generate high-quality data to examine complex cellular mechanisms, advancing cell-based research via lab automation workflows.
In collaboration with Eppendorf®, this study assesses the performance of the Absorbance 96 Automate integrated on-deck with the epMotion® liquid handling system for automating the CytoTox 96® Non-radioactive Cytotoxicity Assay, comparing it with another benchtop microplate reader. This comparative analysis demonstrates comparable optical density (OD) readouts and reproducibility between the Absorbance 96 Automate and the benchtop counterpart, validating its robustness and reliability for automated cell-based assays. The study underscores the potential of the Absorbance 96 Automate as an effective on-deck microplate reader. It offers valuable insights for researchers seeking automated microplate reading solutions in their experimental workflows, particularly cell-based assay research.
Automated Cell-based Assay Workflow
HeLa cells were seeded into a 96-well microplate using the epMotion® liquid handling system. After incubation in a CO₂ incubator, different concentrations of Staurosporine, a potent and cell-permeable inhibitor known for its impact on various protein kinases leading to cytotoxicity (1), were added to the cells through an automated workflow. This was followed by an additional incubation at 37 degrees Celsius in a CO₂ incubator.
To assess induced cellular cytotoxicity, the CytoTox 96® Non-radioactive Cytotoxicity Assay kit from Promega was used. Utilizing the epMotion® liquid handling system, CytoTox 96® substrate and stop buffer were added to the cells according to the manufacturer's protocol. Subsequently, samples were automatically subjected to absorbance measurement at 490 nm using the Absorbance 96 Automate, positioned on the deck of the epMotion® workstation.
Results
Absorbance 96 Automate and GloMax® Discover Microplate Reader Show Comparable Measurement Performance
To assess the performance of the Absorbance 96 Automate, a comparative analysis was conducted with the GloMax® Discover microplate reader (Promega). Staurosporine-treated cell samples were subjected to absorbance measurements at 490 nm using both instruments. The obtained results revealed similar optical density (OD) values across the sample range (0 – 5x10⁴ nM Staurosporine). Also, a high correlation (R²= 0.9997) of the readouts was observed between the two instruments, as depicted in Figure 1.
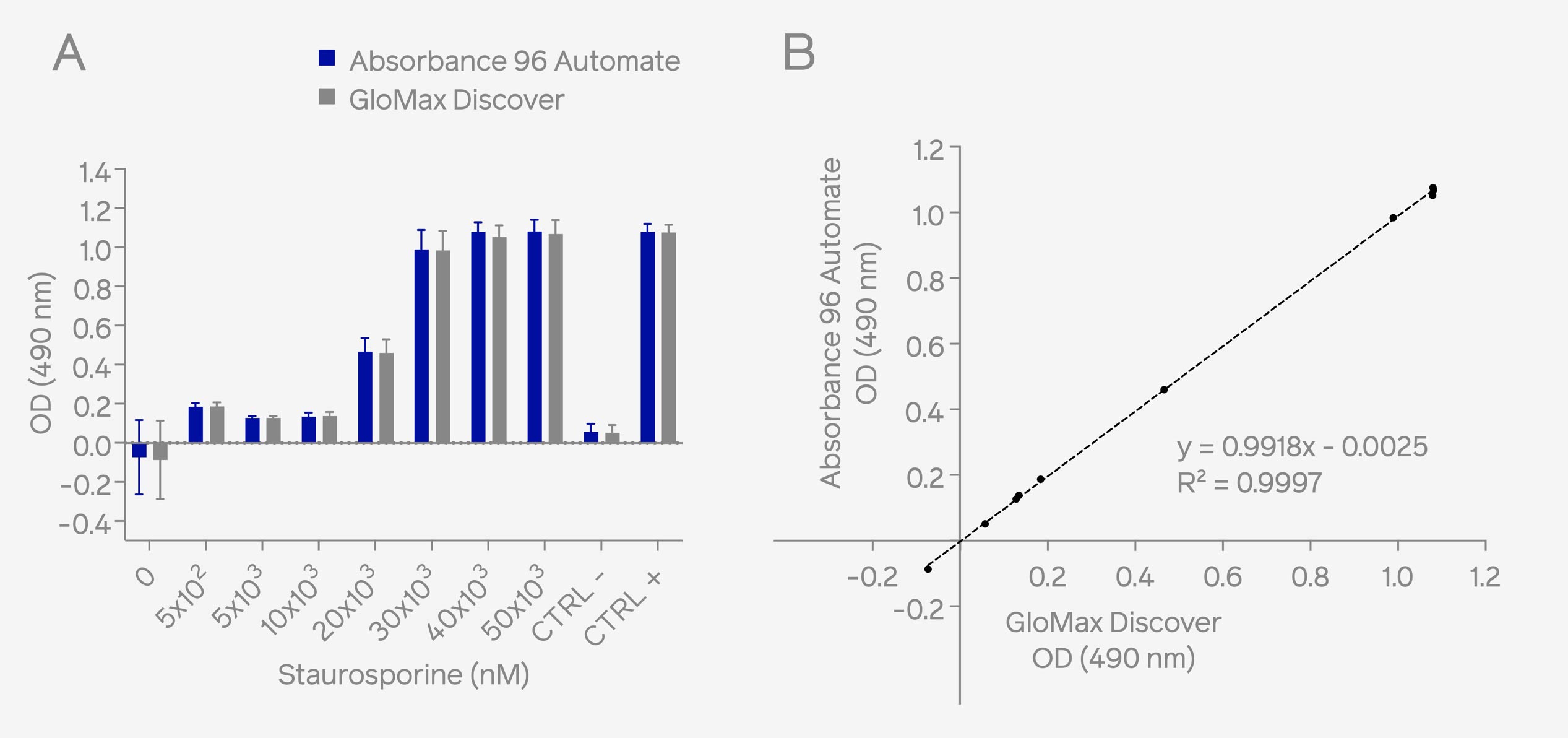
Fig. 1: HeLa cells were treated with Staurosporine to induce cellular cytotoxicity. Assessment of cellular cytotoxicity was conducted using the CytoTox 96® Non-radioactive Cytotoxicity Assay kit. Absorbance measurements at 490 nm were performed using both the on-deck integrated Absorbance 96 Automate on the epMotion® liquid handling system and the benchtop microplate reader, GloMax® Discover. Results demonstrate comparable OD readouts (A) and high correlation (B) across different OD ranges.
The findings affirm that the Absorbance 96 Automate exhibits comparable reproducibility, sensitivity, and linearity within the evaluated dynamic range when juxtaposed with the GloMax® Discover. Notably, the Absorbance 96 Automate showcased a reduced readout time (3 seconds) compared to the GloMax® Discover (52 seconds), underscoring its efficiency in facilitating rapid readouts.
Summary
Integrating the Absorbance 96 Automate onto the epMotion® deck is characterized by its seamless nature. It offers a convenient automatic workflow for evaluating cell-based assays with precision and enabling high-throughput capabilities, emphasizing lab automation. Outcomes from this comparative study confirm the validation of the Absorbance 96 Automate as a reliable and efficient on-deck microplate reader for absorbance measurements in an automated CytoTox 96® cytotoxicity assay, highlighting its potential utility in various other cell-based assay lab automation workflows.
Reference
1. Ding Y. et al. Staurosporine suppresses survival of HepG2 cancer cells through Omi/HtrA2-mediated inhibition of PI3K/Akt signaling pathway, Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2017, 39(3).